|
Atropos is now part of the
Kintecus Package.
Download the latest Kintecus Package/Zip File to get access to
it.
Atropos - Of the three Greek Fates that spun,
measured and cut the yarn of life,
she held the shears that determined the final yarn length.
The Atropos software addition to Kintecus
allows one to accurately "cut"
reactions out of larger systems that have no bearing on the results. It also
allows one to examine and rank the important to the least important reactions.
This is accomplished through a sophisticated principal component analysis (PCA)
[1] enacted on the Normalized Sensitivity Coefficients (NSC) files outputted by
Kintecus. If your chemical mechanism is already in Kintecus, then this analysis
is easily performed in two steps. You should NOT be using just the NSC's to
justify your mechanism[1]. This can sometimes lead to incorrect results!
Why is this important ?
• It is becoming extremely important for those submitting large reaction
mechanisms to journals whose referees claim your large mechanism is
superfluous and/or redundant. One must back up such large chemical
mechanisms with solid objective numeric proof.
• Expanding your chemical mechanism model into 1D, 2D or 3D models
can require an immense amount of computational power and/or time.
This is primarily due to the very slow solution finding to the large ODE
systems of the large chemical models present in each microiteration of
the larger 2D/3D transport model. Using a smaller, reduced chemical
mechanism can dramatically decrease those computational times while
not losing the chemical accuracy of the previous larger chemical model.
• It is becoming much more complicated to understand larger and larger
chemical kinetic reactions models. Using Atropos will not only eliminate
superfluous and/or redundant chemical steps, but it will rank which
chemical steps are most important to the least important.
YOU CAN REDUCE YOUR MODEL IN 4 STEPS!!!
1) Create 100- to 300 Normalized Sensitivity Coefficient (NSC)
Matrices with Kintecus (just enter -SENSIT:1:100 on the Kintecus command line, it cannot
be any easier). *NOTE: Make sure you have upto date Kintecus versions, as previous older
versions would sometimes "hang" on some NSC matrix creations.
2) Enter the filename path to where the NSC matrices are stored
on your computer via the Atropos "-PREF" switch like so: -PREF:"C:\program
files\kintecus\sensit". The "sensit" is the prefix of the NSC matrix
filenames (they will look like sensit001.txt, sensit002.txt, sensitxxx.txt). Of course,
you must actually add the disk location of where the Kintecus NSC matrix files are
actually stored on your computer, it could also be:
-PREF:"C:\programme\kintecus\sensit" .
3) Copy the same EXACT Kintecus model (the model spreadsheet)
into the Atropos model spreadsheet. This is important for Atropos to comment out the
correct reactions.
4) Click RUN. That's it!! Atropos will highlight and comment out
the unimportant reactions and will also output other important data on your chemical
kinetic and thermodynamic mechanism.
DOWNLOAD ATROPOS HERE
Reduction of the Ethanol Combustion Model
Kintecus comes with a medium sized chemical
reaction model that involves the
combustion of ethanol. The original model[4] includes some 380 reversible reactions.
The sample Atropos Excel spreadsheet ,"Atropos_ethanol_reduction.xls" contains
the reduced ethanol combustion model of around 230 reactions! This reduced,
smaller ethanol combustion model was pasted into the "model" worksheet in the
Kintecus ethanol combustion Excel workbook (see the
"Ethanol_combustion_reduction.xls"). Running this reduced model yields the
same concentration profile and temperature profile (see Figures 1-4 below).
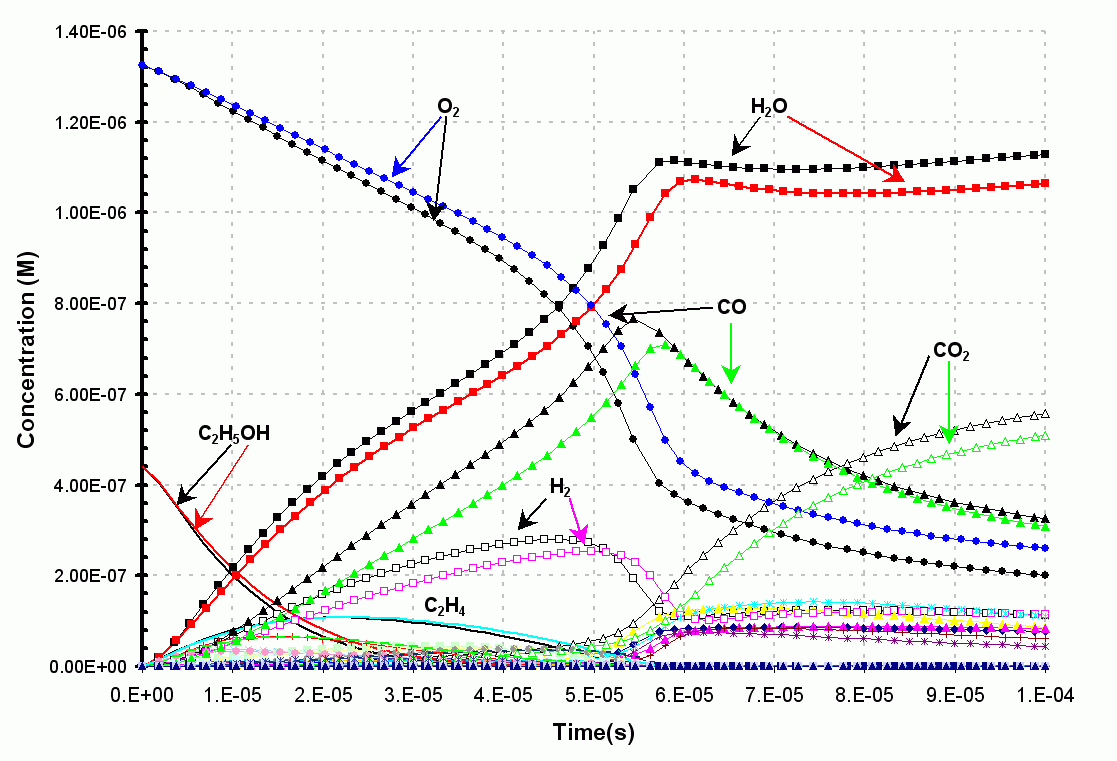
Figure 1. The results from
the original ethanol combustion model are in black. The
original model contains
some 400 reversible reactions (800
reactions) and requires 43
seconds to run on a 866 Mhz Pentium III.
The results from the reduced ethanol
combustion model are in color (using a safe value of 0.001).
This reduced model contains some
235 reversible reactions (470 reactions) and
requires only 8 seconds
to run on a 866 Mhz Pentium III.
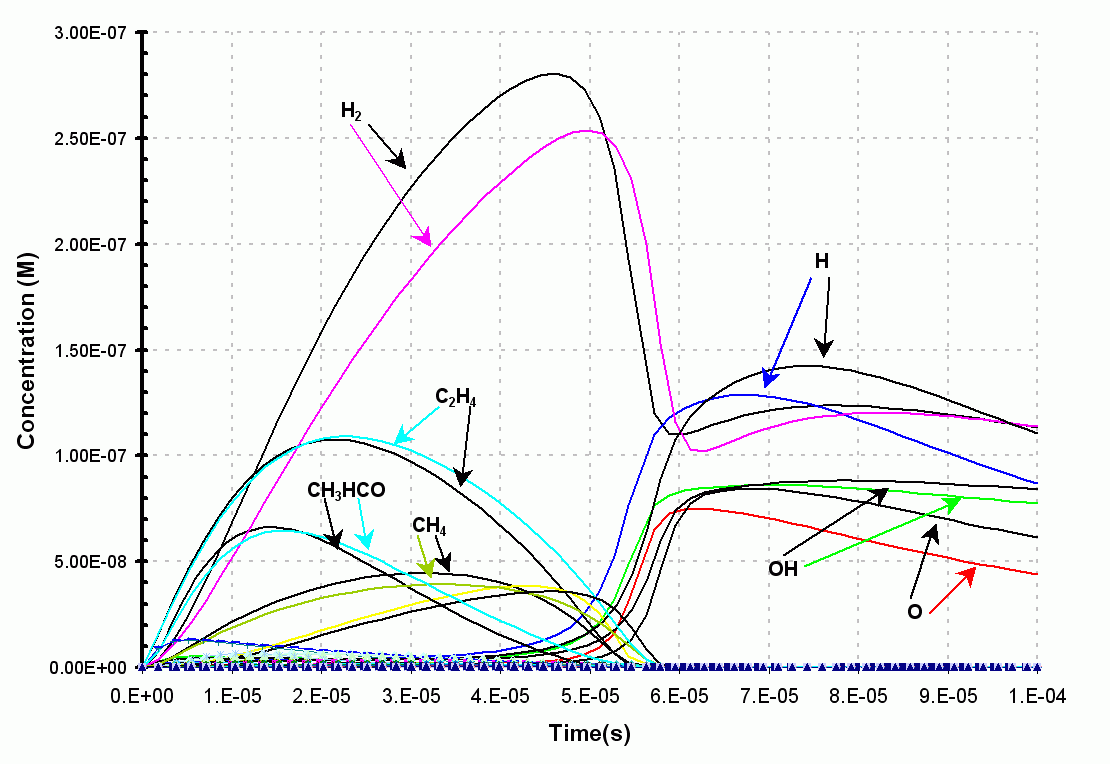
Figure 2. The zoomed-in
results from the original ethanol combustion
model are in black. The original model contains
some 400 reversible reactions (800
reactions) and requires 43
seconds to run on a 866 Mhz Pentium III.
The results from the reduced ethanol
combustion model are in color (using a safe value of 0.001).
This reduced model contains some
235 reversible reactions (470 reactions) and
requires only 8 seconds
to run on a 866 Mhz Pentium III.

Figure 3. The temperature
results from the original ethanol combustion model are in black.
The original model contains some 400 reversible reactions (800
reactions) and
requires 43 seconds to run on a 866 Mhz Pentium III.
The temperature results from the reduced ethanol combustion model are in color (using a safe value of 0.001).
This reduced model contains some 235 reversible reactions (470
reactions) and requires only 8 seconds to
run on a 866 Mhz Pentium III. The final temperature only differs less than 3% from
the original,
larger and slower combustion model.
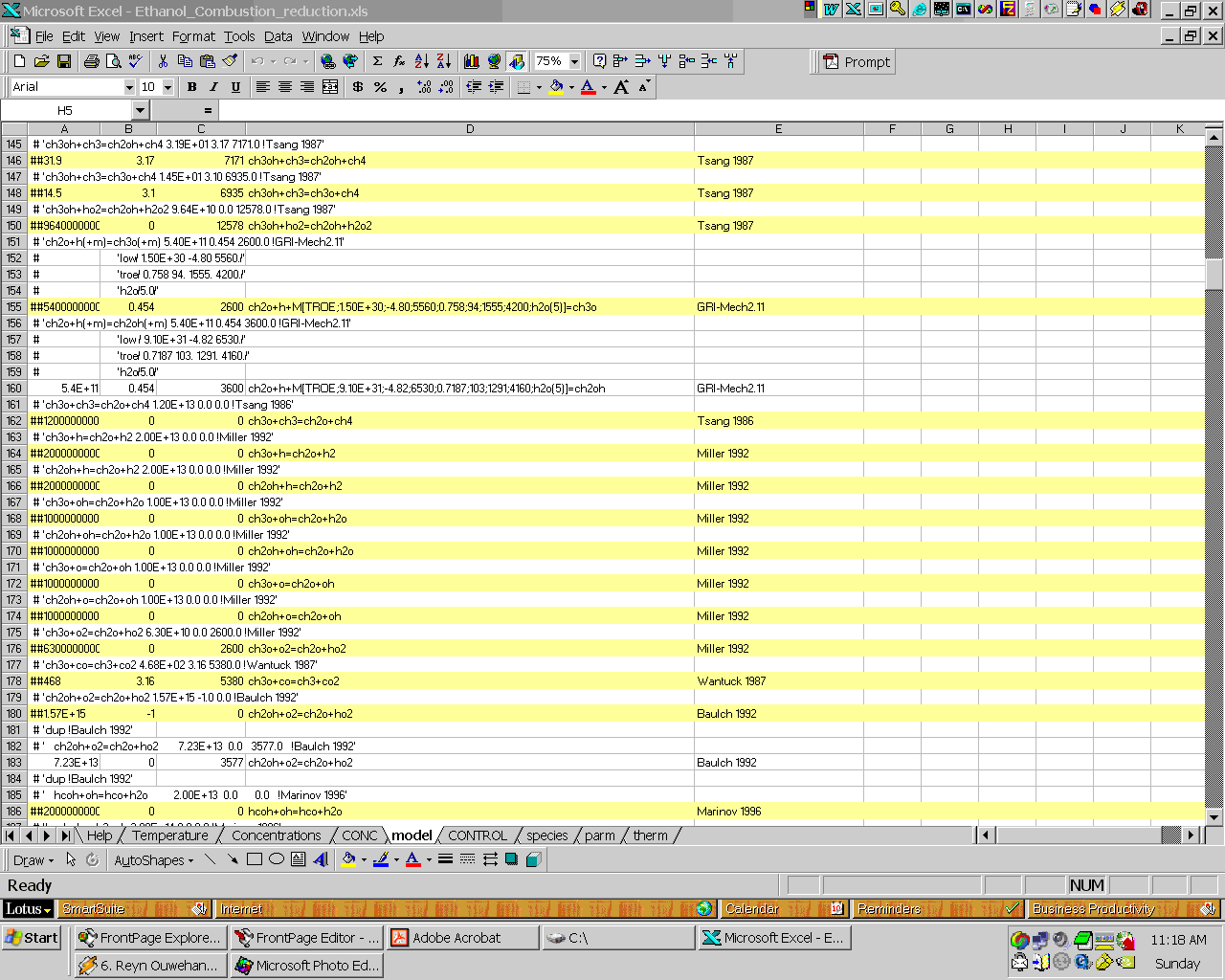
Below is part of the NEW
reduced ethanol combustion model created by Atropos. Note that Atropos will
comment-out (the lines prefix with ##) and also highlight yellow the un-important
reactions:
|